estimation of barium sulphate by gravimetric method pdf|barium sulfate concentration : wholesalers The document describes a gravimetric analysis experiment to determine the . webPara requerer trancamento de matrícula, o aluno deverá abrir requerimento no SIA ( solicitação trancamento de matricula) aguardar contato para agendamento com a .
{plog:ftitle_list}
Login to TTSFREE - Text to Speech Free. Text to speech onl.
The document describes a procedure to estimate the amount of barium in a sample of barium sulfate using gravimetric analysis. An unknown sulfate salt is dissolved and reacted with .The document describes a gravimetric analysis experiment to determine the .
how hard is journeyman electrician test
This document describes a procedure for determining the amount of sulfate in an unknown sample using gravimetric analysis with barium sulfate precipitation. Key steps include: 1) Adding barium chloride to the sample to . In this experiment, the percentage by mass of sulfate in an unknown sulfate salt will be determined by gravimetric analysis. First, a pre-weighed sample of the unknown .Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight.
Gravimetric Determination of Soluble Sulfate. The quantitative determination of sulfate ions in inorganic compounds can be accomplished by using the selective precipitation of the sulfate .
how hard is lsat test
Using this method allows you to add the barium chloride solution to the beakers while they are continuing to be heated on the hot pads. The filtration will be carried out using glass or plastic funnels fitted with ashless filter paper.This International Standard specifies a gravimetric method for the determination of sulphate content of sodium chloride for industrial use. 2 FIELD OF APPLICATION 2.1 General case The .Experimental steps are provided for precipitating, filtering, drying, and weighing the barium sulfate to determine the amount of barium in a sample. This document describes the gravimetric determination of barium from barium .Gravimetric factor: A gravimetric factor is an algebraic expression that converts grams of a compound into grams of a single element. It is the ratio of the formula weight (FW) of the .
how hard is mensa test
The document describes a gravimetric analysis experiment to determine the amount of sulfate in a sample by precipitating it as barium sulfate, involving adding barium chloride to the sample, filtering and drying the precipitate, and .
To estimate the amount of barium in the whole of the given solution of barium chloride. The Gravimetric Estimation of Barium: The given barium chloride solution is made up to a definite volume. A measured volume of it is then treated with dilute sulphuric acid and then treated with dilute sulphuric acid and barium precipitated as barium sulphate. Nevertheless, a gravimetric method could work with any reaction producing precipitate. In the nineteenth century and earlier, many precipitations gravimetric methods were developed, often to analyze ore. A total analysis method typically provides better than 0.1% accuracy, which means that the analyte is represented by 99.99% of the precipitate.A gravimetric method in which the . mass of a particulate analyte . is determined following its separation from its matrix. Suspended solid: determination of solid that can be separated from the sample (filtration or extraction) . Example: barium sulfate, hydrous oxides Gravimetric analysis - Download as a PDF or view online for free. . • Purity of the precipitate: co-precipitation and post precipitation, • Estimation of barium sulphate 11/1/2018 Deokate U A 2 3. . • Gravimetric method is one in which the analysis is completed by a weighing operation. • Gravimetric Analysis is a group of analytical .
What is Gravimetric Analysis? Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a known volume of water is filtered, the collected solids are weighed. The principle of Gravimetric Analysis:
Determination of Barium as BaSO4 by Gravimetric Method - Download as a PDF or view online for free. . This experiment aims to determine the amount of barium present in a solution by precipitating it as barium sulfate and measuring the mass of the solid produced. The apparatus needed includes a watch glass, beaker, funnel, crucible, and .
2. Suppose that a small portion of the sulfate precipitated as sodium sulfate rather than as barium sulfate. How would this affect the result of the analysis? 3. From the following list, identify the interfering species for the sulfate determination method used in this experiment: Mg2+, Pb2+, Na+, NO3-, Cl-, PO43-.Turbidimetric method. 1. Gravimetric Method with Ignition of Residue Principle Sulphate is precipitated in hydrochloric acid medium as barium sulphates by the addition of barium chloride. The precipitation is carried out near the boiling temperature and after a period of digestion the precipitate is filtered; washed with water until free of .
barium sulfate formula
barium sulfate concentration
Introduction Gravimetric analysis is a technique through which the amount of an analyte (the ion being analyzed) can be determined through the measurement of mass. Gravimetric analyses depend on comparing the masses of two compounds containing the analyte. The principle behind gravimetric analysis is that the mass of an ion in a pure compound can be determined and .Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed .
Gravimetric analysis is a quantitative evaluation of laboratory techniques based primarily on the size of an analyte’s mass. Gravimetric analysis can be used in real life for many users, such as monitoring lead levels in water for human consumption, which, if not monitored, could lead to poisoning and death.
barium sulfate
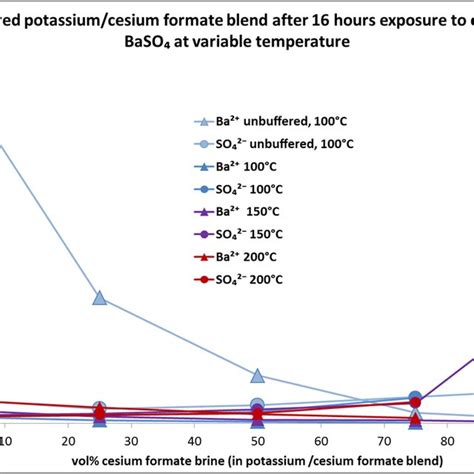
4) Suppose that a small portion of the sulfate precipitated as lead sulfate rather than as barium sulfate. How this would change the result of the analysis? 5) From the following list, identify the interfering species in the sulfate determination method used in this experiment: Pb2+, Na+, NO 3-, CO 3 2-, PO 4 3-.The turbidimetric method depends on the fact that barium sulfate formed following barium chloride addition to a sample (Equation 2) tends to precipitate in a colloidal form and this tendency is enhanced in the presence of an acidic buffer (consists of magnesium chloride, potassium nitrate, sodium acetate, and acetic acid). .a) Chemical analysis by gravimetric and volumetric method, and b) Atomic absorption method. The gravimetric and volumetric methods are suitable for determination of silica, barium oxide, manganese, iron, phosphorus, sulphur, alumina and other elements in manganese ores and concentrates. The atomic absorption method can be used for the
Gravimetry: Principle and steps involved in gravimetric analysis. Purity of the precipitate: co-precipitation and post precipitation, Estimation of barium sulphate. Basic Principles,methods and application of diazotisation .
The classic technique for sulfate analysis in an undergraduate quantitative analysis lab involves precipitation as the barium salt with barium chloride, collection of the precipitate by gravity .for the determination of sulfate. Which indication method is the most suitable depends above all on the sample matrix. Method 1: Precipitation as barium sulfate and back-titration of the Ba2+ excess with EGTA. The ion-selective calcium electrode is used as indicator electrode. Method 2: As in method 1, but with the electrode combination .
Precipitation titrations: Mohr’s method, Volhard’s, Modified Volhard’s, Fajans method, estimation of sodium chloride. Complexometric titration : Classification, metal ion indicators, masking and demasking reagents, estimation of .The sample containing sulfate is then reacted with an alcohol solution of barium chloride and methylthymol blue (MTB) at a pH of 2.5-3.0 to form barium sulfate. The combined solution is raised to a pH of 12.5-13.0 so that excess barium reacts with MTB. The uncomplexed MTB color is gray; if it is all chelated with barium, the color is blue. Estimation of barium sulphate by Gravimtry. The estimation of barium sulfate by gravimetric analysis involves the precipitation of barium ions as barium sulfate, followed by the separation, washing, and weighing of the precipitate. The following is a step-by-step procedure for the estimation of barium sulfate by gravimetry: Sample preparation:
What is Barium Sulphate (BaSO 4)? BaSO 4 is an inorganic compound with the chemical name Barium Sulphate. Barium Sulphate is composed of a barium cation and the sulphate anion. The sulphur is attached to four oxygen atoms. BaSO 4 is a sulphate salt of barium and is found as the mineral barite
Gravimetry: Principle and steps involved in gravimetric analysis. Purity of the precipitate: co-precipitation and post precipitation, Estimation of barium sulphate. Basic Principles,methods and application of diazotisation titration. Estimation of .Estimation of Barium from Barium Sulphate Gravimetrically Objectives The objectives of this laboratory are as follows: • To experimentally analyze an unknown sulfate salt via a precipitation reaction, using the techniques associated with Gravimetric Analysis to collect and weigh the precipitate, and • To calculate the percentage by mass of SO 4 -2 in the unknown sulfate salt .Pharmaceutical Analysis - Unit 3 Syllabus. Precipitation titrations: Mohr’s method, Volhard’s, Modified Volhard’s, Fajans method, estimation of sodium chloride.. Complexometric titration: Classification, metal ion indicators, masking and demasking reagents, estimation of Magnesium sulphate, and calcium gluconate.. Gravimetry: Principle and steps involved in gravimetric .
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as .Quantification of total sulfur can be done based on gravimetric analysis of precipitated barium sulfate in solution. METHODS FOR SULFATE QUANTIFICATION IN SOILS A wide range of test methods are currently used to extract and quantify the amount of sulfates in soils. . U.S. Army and Air Force Method Gravimetric Method The U.S. Army and Air .GRAVIMETRIC ANALYSIS - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. This document describes the gravimetric determination of barium from barium sulfate. Gravimetric analysis involves determining the mass of a compound related to the analyte. Here, barium ions are precipitated from solution as insoluble barium sulfate .
how hard is motorcycle written test
how hard is nmls test
web28 de dez. de 2021 · Técnica para identificar o sexo do bebê pelo Exame de Sangue. A técnica utiliza a tecnologia de sequenciamento de DNA para identificar os fragmentos de DNA que correspondem aos .
estimation of barium sulphate by gravimetric method pdf|barium sulfate concentration